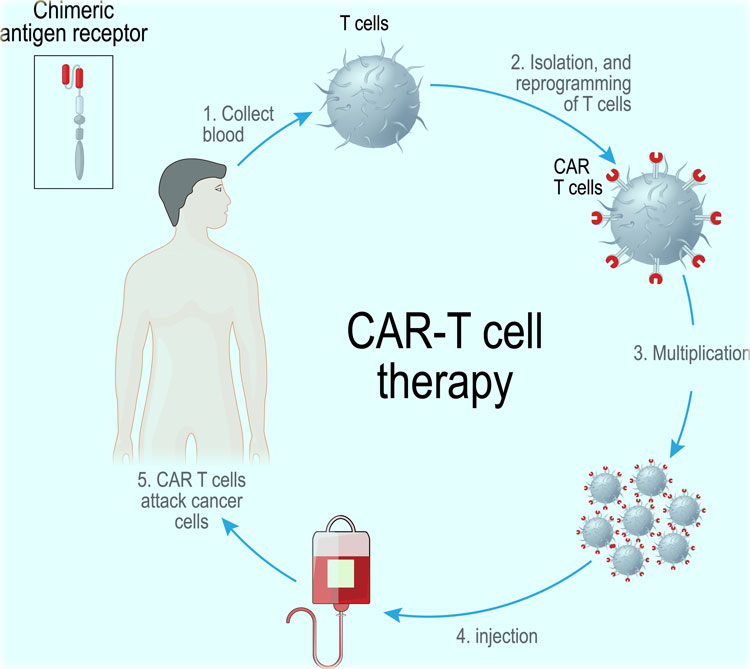
India’s first Chimeric antigen receptor T-cell (CAR-T) treatment was conducted at the bone marrow transplant unit at the ACTREC, Tata Memorial Center (TMC) in Mumbai, Maharashtra. The clinical trial is a joint venture between the Indian Institute of Technology (IIT) Bombay and the Tata Memorial Center.
*The clinical trial of CAR-T was supported by Biotechnology (DBT) through the National Biopharma Mission (NBM) – Biotechnology Industry Research Assistance Council (BIRAC).
*The Government of India has approved Rs. 19.15 crore to the National Biopharma Mission-Brock Group to conduct the first phase of the human phase-1/2 clinical trial of CAR-T cells.
*CAR-T treatment worldwide shows promising results in the final stages of cancer patients, especially patients with severe lymphocytic leukemia.
*CAR-T cell therapy per patient costs Rs 3-4 crore and this technology is not available in India.
*Now to overcome this challenge this technology is being developed at low cost and made available to patients in India.
CAR-T at India:
*BIRAC and DBT have taken various initiatives over the last 2 years to improve CAR-T cell technology against cancer and they are partially supported by the PACE-BIRAC project.
*The CAR-T cells were designed and manufactured by Rahul Purwar, Professor, Department of Biology, Biology and Bioengineering (BSBE), IIT Bombay, and his team.
*The clinical trial was conducted by a TMC team led by Dr. Gaurav Narula, Professor of Pediatric Oncology and Health Sciences.
